
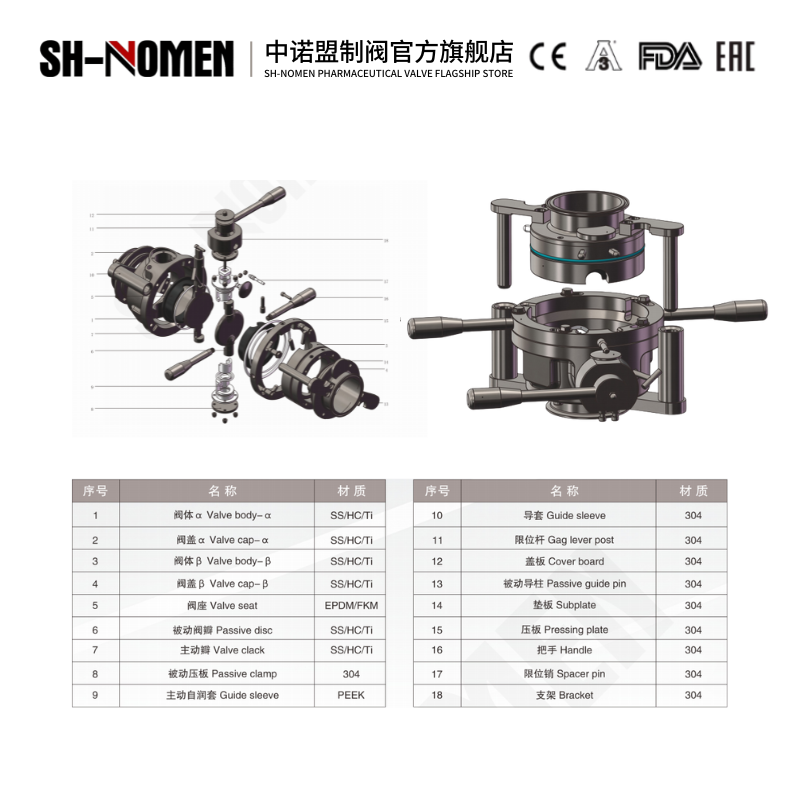
Working Principle
The aseptic inlet valve is a diaphragm- or bellows-sealed shut-off valve designed for sterile process streams. When the actuator retracts the stem, the diaphragm/bellows lifts off the weir seat, opening a full-bore path that allows sterile liquid or powder to enter the reactor or vessel. In the closed position, the actuator compresses the diaphragm/bellows against the seat, creating a hermetic seal that isolates the process side from the external environment while maintaining system sterility. Steam or sterile barrier fluid can be supplied to the valve body for SIP (Sterilize-In-Place) cycles.
Key Features
1. Absolute sterility: diaphragm or bellows isolates product from atmosphere; all wetted parts are 316L/EN 1.4435 with Ra ≤ 0.4 µm finish.
2. SIP/CIP capability: valve withstands steam at 121–134 °C for sterilization and allows full CIP cleaning without disassembly.
3. Zero dead-leg design: minimizes product hold-up and ensures complete drainability, meeting ASME BPE and FDA requirements.
4. High sealing integrity: elastomeric or PTFE diaphragms provide bubble-tight shut-off, preventing cross-contamination.
5. Modular actuation: manual handwheel, pneumatic or electric actuators are interchangeable for flexible automation.
6. Traceability: each valve is supplied with material certificates and a unique serial number for full batch tracking.