See more information about SH-NOMEN and learn more about the use of valves

The Heart of Biopharmaceuticals

In the pharmaceutical industry,
every vaccine dose and every innovative pill is born under ultra-strict cleanroom conditions.
Valves—functioning as the “throat” of hygienic piping systems,
directly determine drug quality and production safety.
Drawing on deep expertise and breakthroughs in forged valves,
SH-NOMEN delivers end-to-end solutions that span from laboratory scale to full commercial production,
serving as the core force driving the upgrade of China’s pharmaceutical equipment worldwide.

From the laboratory to the production line, corrosion resistance is the lifeline.

Design: zero-dead-leg construction—leaves contamination nowhere to hide.

Sealing: a precision game measured in millimeters.
A single leak can spell disaster. Our forged valves employ a high-purity PTFE + EPDM dual-seal system that cuts friction and elevates sealing integrity. For lyophilized powder filling, the 90° fast-actuation design delivers exact flow control, eliminating any risk of drips or spills.

Compliance: backed by global certifications—the “international passport” to quality.
From FDA and EAC to ASME BPE and ISO 15848-1, forged valves must clear every hurdle. Case in point: SH-NOMEN’s HC276 Hastelloy ball valve meets ASTM B265, ensuring full traceability from design to delivery.

Myth 1: “Forging is always more expensive than casting?”
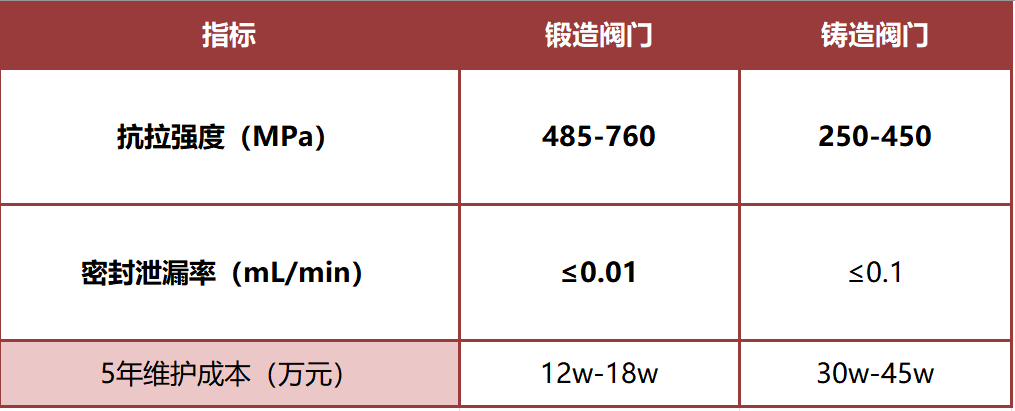
→ Reality: The up-front price may be slightly higher, but total cost of ownership drops by ~40 %. With SH-NOMEN’s in-house forging expertise and integrated supply chain, our forged valves often undercut the price of cast equivalents—even when compared with Tier-1 international brands—delivering superior quality while driving down cost and risk.
The market typically commands a hefty premium for forged valves. By mastering core forging technologies and continuously refining our processes, SH-NOMEN has eliminated this surcharge. We choose to forgo excessive margins—not to undercut value, but to put superior forged valves within reach of industry leaders and advance the sector as a whole.

Case 1: Doubling WuXi Biologics TIDES Capacity

SH-NOMEN sterile valves are certified to FDA, 3A, and ISO 14644 standards and are already qualified suppliers to global top-20 pharmaceutical companies such as Pfizer and AstraZeneca.
With the commissioning of our new 50,000 m² facility in Ruian Coastal New District in 2025, annual capacity will reach 500,000 units, positioning us to capture a significant share of China’s high-end sterile-valve market.

数据来源2024-北京博研咨询调研报告
Amid the explosive growth of China’s CDMO sector
projected to reach RMB 1571 billion by 2025
SH-NOMEN is executing a three-pillar strategy:
technology autonomy, intelligent manufacturing, and global service.
We continue to deliver cost-leading solutions that empower pharmaceutical partners across the value chain.
Whether pioneering synthetic biology or enabling precision manufacturing for gene therapies,
SH-NOMEN forged valves stand as the benchmark for quality and innovation.
